Turkish Researchers 3D Print Artificial Cornea For Transplantation
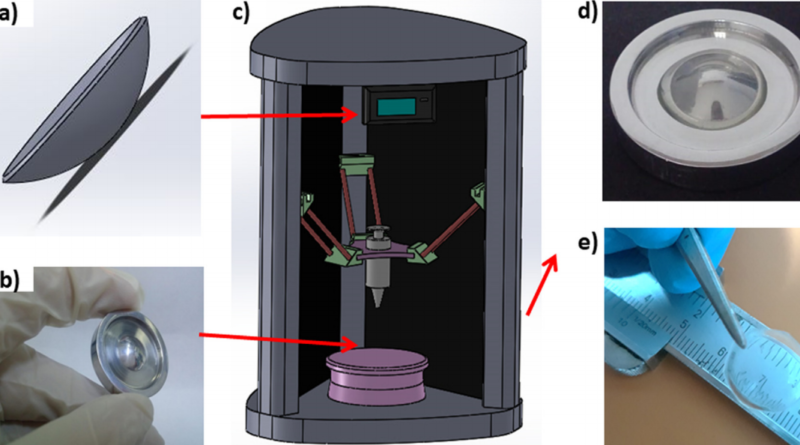
A team of researchers from Marmara University, Turkey have 3D printed an artificial cornea suitable for transplantation. With the help of an aluminum mold, an FFF 3D printer was used to fabricate a PVA-chitosan corneal construct with the light bending properties of the real thing.
Preliminary biostability studies revealed that the composite structures were compatible with human stem cells, encouraging them to differentiate into stromal cells. The results of the study indicate great potential for the rapid and custom fabrication of cornea constructs for clinical applications.
With the aim of finding a sustainable alternative to cornea donations, the team first combined chitosan and PVA to form a biocompatible, elastic, permeable-to-oxygen composite material for the corneas. The researchers then drafted up an aluminum mold in SolidWorks and machined it before any 3D printing took place. The mold was used to perfect the cornea shape as 3D printing with FFF technology alone wouldn’t give them the dimensional precision they needed. Then, with varying proportions of chitosan in the mix, a set of sample cornea constructs were fabricated.
The 0.4mm thick constructs were subjected to scanning electron microscopy and UV spectrophotometry to determine their optical properties, with further biocompatibility tests with human stem cells determining clinical feasibility. The results showed that the PVA corneas had the correct size and shape for precise light refraction, and light transmittance values were found to decrease with additional chitosan content. Tensile strength also decreased with chitosan content but the constructs could all comfortably support the typical intraocular pressure.
The real potential of the study comes from the biocompatibility test, however, as the printed structures were compatible with the stem cells even after 30 days of degradation. With the appropriate nurturing, the stem cells could differentiate into a usable stroma layer, supporting the natural healing process of the body post-transplantation. The team concluded that their work, with further developments, could lead to a more safe and sustainable method of corneal blindness treatment.
The 3D printing of medical devices and implants has skyrocketed in recent years, although FFF is rarely the technology used. Chemical company Evonik is looking to change that as it has recently launched its new Vestakeep i4 3DF implant grade PEEK filament into its portfolio of 3D printing materials. The new filament meets ASTM F2026 standards and will be used to produce surgical implants.