Researchers Develop Nano Engineered Bio Ink to 3D Print Functional Bone Tissue
Scientists in the Department of Biomedical Engineering at Texas A&M University are seeking to advance the field of 3D bioprinting functional tissues, by conducting research into the development of new biomaterials.
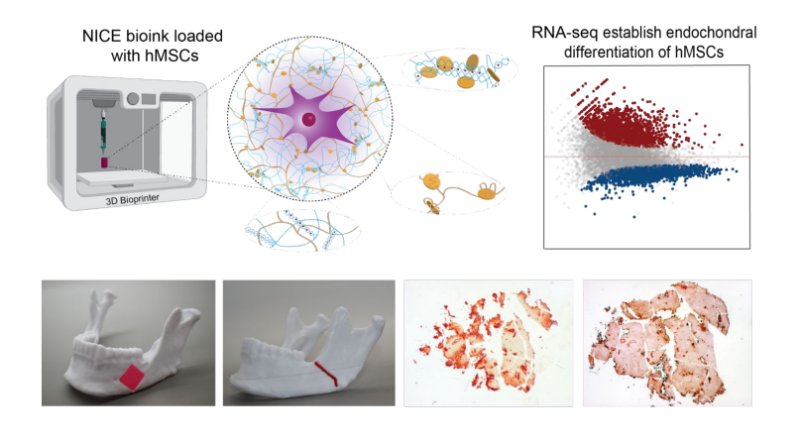
Dr. Akhilesh K. Gaharwar, an associate professor in the department, has created a highly 3D printable bioink, which can be used as a platform for generating anatomical-scale functional tissues. The new material developed by Gaharwar’s research group, known as Nanoengineered Ionic–Covalent Entanglement (NICE) bioink, has been designed to overcome the deficiencies of current bioinks in relation to structural stability. Commenting on the benefits of the NICE bioink, Gaharwar states: “The next milestone in 3D bioprinting is the maturation of bioprinted constructs toward the generation of functional tissues.”
In their study, Gaharwar’s research group first outlined the emergence of 3D bioprinting as a technique for fabricating patient-specific, implantable constructs for regenerative medicine. Using hydrogels and combining them with cells and growth factors, these bioinks are 3D printed to create tissue-like structures intended to imitate the function of natural tissues.
One particularly useful application of the technology is in patient-specific bone grafting, a surgical procedure that replaces missing bone in order to repair bone fractures. As traditional treatments for managing bone defects and injuries are slow and expensive, Gaharwar states that developing replacement bone tissues with bioprinting could create exciting new treatments for patients. These can be used to treat defects and conditions such as arthritis, bone fractures, dental infections and craniofacial defects.
Recent advancements in the field have come from Rice University and the University of Maryland (UMD). Scientists at these institutions have outlined a new proof-of-concept for 3D printing artificial bone tissue to help repair damage related to arthritis and sporting accidents.In their tests, the researchers found that the enclosed cells began depositing new proteins containing a cartilage-like extracellular matrix that subsequently calcifies to create a mineralized bone over a three-month period. Five percent of these 3D bioprinted scaffolds consisted of calcium, which is similar to cancellous bone, the network of spongy tissue typically found in vertebral bones.
Gaharwar’s research group used a genomics technique called whole transcriptome sequencing (RNA-seq) to examine how these bioprinted structures were able to induce stem cell differentiation. RNA-seq works by capturing a snapshot of all genetic communication inside the cell at a given moment. The team worked with Dr. Irtisha Singh, assistant professor at the Texas A&M Health Science Center, who served as a co-investigator.Using their bioink and research results, Gaharwar’s team plans to demonstrate in vivo functionality of the 3D bioprinted bone tissue.
Source: https://3dprintingindustry.com/